Cascais, Portugal
2022 Vallee Summer Symposium

The 2022 Vallee Summer Symposium took place in the lovely seaside town of Cascais, Portugal, from June 10 to 13. Some 90 delegates convened at the hotel Grande Real Villa Itália overlooking the Atlantic Ocean, which provided a magnificent setting with vistas far out to sea and a fabulous terrace complete with preening peacock. Offshore breezes kept the temperature several all-important degrees cooler than Lisbon or further inland – and the science wasn’t bad either!
Check out the pictures in the photo gallery below the peacock!
The symposium was divided into three sessions chaired by Vallee Visiting Professors Lewis Cantley (Harvard Medical School), Eva Nogales (University of California, Berkeley), and Ernst Winnacker (Vallee Foundation Director). Ten Vallee Visiting Professors, two Vallee Scholars, and one guest lecturer from our host country generously shared their recent research which prompted some terrific discussions in the Q&A sessions as well as over drinks and the excellent meals.
Our first speaker, Dr Elaine Fuchs (Rockefeller University), a pioneer in skin biology and associated human genetic disorders presented work from her lab entitled “Stem Cells: Coping with Stress,” that highlighted recent research which explored the idea of inflammatory memory in stem cells. Establishing memory requires an inflammation-triggered transcription factor able to recognize and bind to nucleosome repressed chromatin, and AP1 factors of the FOS/JUN superfamily, which in other systems act in chromatin remodeling and transcriptional activation. Once established, stem cell transcription factors gain access to the opened memory domains as do histone marks such as H3K4me1, such that after inflammation has subsided and the inflammation-triggered transcription factors and FOS are no longer present, the chromatin can still be maintained in a memory state---accessible but associated with genes whose transcription is silent/suppressed. For the memory to be recalled and the genes to be activated, all that is needed is the general stress inducer FOS, since the chromatin is still in an open state.
Next, Vallee Scholar Dr Tanmay Bharat (MRC Laborary of Molecular Biology, Cambridge) presented recent research using electron tomography, combined with several structural and cell biology techniques, to study cell surfaces of prokaryotes at the atomic level. Surface molecules mediate cellular interactions with the environment and play important roles in key processes including cell adhesion, biofilm formation and antibiotic tolerance in pathogenic bacteria. His research aims to push the boundaries of cellular structural biology, with technical developments aimed at identifying molecules on cell surfaces and resolving their high-resolution details in prokaryotic cells. As an example, Tanmay talked about how surface molecules allow pathogenic bacteria such as P. aeruginosa to evade antibiotics with biofilm formation.
Gene regulation is central to all biology. RNA molecules, with their ability to both encode information and exert catalytic activities, play a key role in the regulation of gene expression. Our guest lecturer, Dr Maria Carmo-Fonseca (IMM, Lisbon), presented research from her lab which shows how non-coding genetic variation influences RNA splicing and contributes to human disease. The lab is combining gene editing approaches with reprogramming patient-derived iPSC and inducing their differentiation into cardiomyocytes to identify aberrant splicing patterns caused by mutations detected in patients with hypertrophic cardiomyopathy and develop personalized approaches to correct mis-splicing.
Over the past decade, research into the biology and applications of CRISPR-Cas systems has become one of the most dynamic and rapidly developing areas in the life sciences, holding great promise for future biotechnological and biomedical developments. Dr Emmanuelle Charpentier (Max Planck Institute for Pathogen Research) took us back to her discovery of the CRISPR-Cas9 mechanism and its use as a revolutionary genome engineering technology. In her talk "CRISPR: Harnessing Nature for Genome Engineering", she discussed the biological role, mechanisms and evolution of CRISPR-Cas immune systems, and discussed recent aspects and developments of the CRISPR-based genome engineering in life sciences and medicine.
Dr Kay Davies (University of Oxford) reported on the “Challenges of Genetic Therapies for Duchenne Muscular Dystrophy,” a devasting muscle-wasting disease affecting boys who become wheelchair bound around 12 years of age and who survive until their early twenties. One approach to therapy is to increase levels of the protein utrophin which is very similar to the missing dystrophin. This strategy has shown efficacy in preventing the disease in animal models. A clinical trial in patients with the drug ezutromid showed some promise but the drug is rapidly metabolised Armed with the knowledge of the mechanism of action of ezutromid, the Davies group, in collaboration with the chemists led by Russell in chemistry at Oxford, are working on a new drug series with better drug-like characteristics than ezutromid. This approach should treat all patients whatever their underlying mutation.
“Genetic and phylogenetic analysis of asparagine to aspartic acid protein editing of N-glycosylated proteins by the NGLY1 deglycosylase in C. elegans and viruses” was the title of the talk given by Dr Gary Ruvkun (Harvard Medical School.) Many viral proteins are secreted via the endoplasmic reticulum where they are N-glycosylated at NxS/T sites. Some N-glycosylated asparagines are then deglycosylated by NGLY1/PNG-1 which also deamidates the N-glycosylated asparagine to aspartic acid, thus editing the target protein sequence. The key client protein for NGLY1/PNG-1 deglycosylation and N to D protein editing was revealed by genetic analysis of C. elegans proteasome regulation to be the endoplasmic reticulum-transiting SKN-1A transcription factor, the major transcriptional regulator of proteasomal degradation capacity. NGLY1/PNG-1 edits N-glycosylated asparagines to aspartic acid on the intact SKN-1 protein as it is retrieved by ERAD from the ER to in turn activate the transcription of target proteasomal genes. The normal requirement for NGLY1/PNG-1 editing of SKN-1A can be bypassed by a genomic substitution of N to D in four NxS/T N-glycosylation motifs of SKN-1A. Strikingly, an analysis of cancer cell genetic dependencies for growth revealed that the mammalian orthologue of SKN-1A, NRF1 is required by a highly correlated set of cell lines as NGLY1/PNG-1, showing that NGLY1/PNG-1 and NRF1 act in the same pathway in vertebrates as well. Analogous N to D substitutions in NxS/T N-glycosylation motifs can be observed in phylogenetic comparisons of SKN-1A between nematode species or NRF1 between disparate vertebrates. NGLY1/PNG-1 has been implicated in viral immunity in mammalian cell culture as well as in human pedigrees. Because N to D edited peptides are clearly produced by NGLY1/PNG-1, and are presented by mammalian HLA, such peptides may activate T-cell killing or B-cell maturation to mediate more robust viral immunity.
Dr Susan Gottesman (NIH, National Cancer Institute), opened the Sunday morning session by reporting on “Multiple Levels of Regulation of Bacterial Stress Responses.” General stress responses in bacteria such as E. coli are mediated by the sigma factor RpoS and lead to resistance to multiple stresses, regardless of the specific inducing signal. Susan has been studying the mechanisms for post-transcriptional induction of RpoS and recovery from stress. Small RNAs positively regulate RpoS translation by opening an inhibitory hairpin; cells rapidly recover via turnover of the small RNAs. Anti-adaptor proteins protect RpoS from proteolysis; our recent work suggests that one of these is part of a complex feedback loop that allows rapid recovery of RpoS degradation once the inducing stress is gone.
Dr Gunnar von Heijne (Stockholm University) talked about cotranslational protein folding. Thanks to the development of a range of new methods this field has seen renewed interest in the past 10 years, and the folding of soluble proteins as they emerge from the ribosome can now be followed almost on the atomic scale. It has also become possible to track the membrane insertion and cotranslational folding of integral membrane proteins on a residue-by-residue level, opening up a new window on membrane protein biogenesis.
Dr Nicholas Tonks and his colleagues at Cold Spring Harbor Laboratory take a multidisciplinary approach to study the structure, regulation, and function of the protein tyrosine phosphatase (PTP) family of enzymes, to illustrate their fundamental importance to the control of signal transduction under normal and pathophysiological conditions. As functional studies have established links to disease, the PTPs have been garnering attention as potential therapeutic targets; however, they remain a largely untapped resource for drug development. A focus of Nick’s lab is PTP1B, the prototypic member of the PTP family that Nick discovered ~30 years ago. Now, detailed understanding of the structure and function of PTP1B is revealing new approaches to the development of small molecule drug candidates.
Dr Martin Lohse (ISAR Bioscience Institute), whose research focuses on the role of receptors in cell signaling and in (patho-)physiology and on the mechanisms of their activation and inactivation, gave a talk entitled “Receptor Signaling in Nanometer-sized Cell Compartments.” Martin reported the discovery that in contrast to long-held opinion the intracellular messenger cAMP is not freely diffusible inside cells, but at basal concentrations is essentially completely bound. This allows the establishment of steep concentration gradients over very short distances. Therefore, stimulation of receptors by low concentrations of hormones, neurotransmitters or drugs causes very local signals, limited to only tens of nanometers. This allows cells to process many signals simultaneously and to act “like chips, not like simple switches”.
Vallee Scholar Dr Darcie Moore (University of Madison School of Medicine and Public Health) presented her work on adult neurogenesis, the production of new neurons in the brain throughout life. To produce neurons, neural stem cells (NSCs) first must exit a quiescent state, the rate-limiting step of neurogenesis, yet there is much unknown about quiescent NSCs due to the limitations of current technologies. Darcie presented her work developing a novel technique using fluorescence lifetime imaging of endogenous fluorescent signals in adult NSCs to identify quiescent cells as well as their transition into an activated state, setting up a means of gathering critical information about the changes that limit stem cells' ability to exit quiescence in aging and disease.
Dr Tyler Jacks (Massachusetts Institute of Technology) is a world leader in the field of cancer genetics and is known for his ground-breaking work on the development of genetically-engineered mouse models of cancer (GEMMs). His talk, “Molecular Dissection of Tumor Evolution,” reviewed a number of recent advances from his group, including new models for studying the intricacies of tumor-immune interactions in the setting of lung and pancreas cancer. He also discussed a novel strategy for identifying potential antigens presented specifically on the surface of cancer cells in such models, involving a conditional, affinity-tagged MHCI protein.
The final talk was given by Dr Karen Vousden who is Cancer Research UK's Chief Scientist and a Group Leader at the Francis Crick Institute. Karen’s research has made contributions to our understanding of the regulation and function of the tumor suppressor protein p53 and how metabolic alterations can contribute to cancer progression. Her talk described the unexpected finding that high intake of the sweetener sucralose can dampen T-cell mediated responses. As a result, sucralose feeding was able to mitigate T-cell-dependent autoimmune disorders in mouse models.
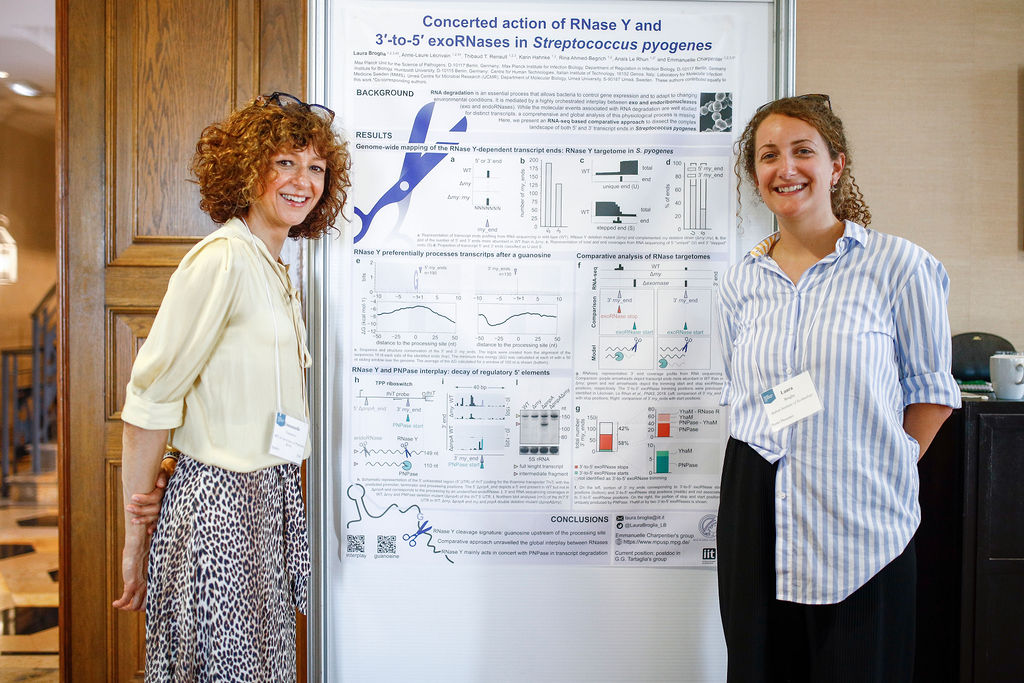
In addition to the scientific talks a lively poster session was held on Sunday afternoon at which eight trainees presented their recent research.
• Anna Bieber (Max Planck Institute of Biochemistry): New insights in yeast autophagy from correlative in situ cryo-ET
• Laura Broglia (Italian Institute of Technology): Concerted Action of RNase Y and 3'-to-5' exoRNases in Streptococcus pyogenes
• Cristina Capitanio (Max Planck Institute of Biochemistry): Correlative approaches for in situ structural study of autophagy in iNeurons
• Maira Di Tano (Weill Cornell Medicine): BRCA2-deficiency alters pancreatic and colorectal cancer metabolic state and sensitivity to high-dose Vitamin C
• Zackery Ely (Massachusetts Institute of Technology): A prime editor mouse for modeling a broad spectrum of somatic mutations
• Paul Sauer (University of California, Berkeley): A novel epigenetic mechanism for controlling gene expression: a study on the protein PRC2 visualized with cryo-EM
• Samantha Sedor (Harvard Medical School): Recognition of Orphan Ribosomal Subunit Proteins by the E2/E3 Hybrid Enzyme UBE2O
• Ines Tomaskovic (Goethe University, Institute for Biochemistry II): The role of SPRTN in resolution of DNA protein crosslinks and replication stress
A chance to get out of the meeting room was provided by an excursion to the National Palace of Queluz on Saturday afternoon. Delegates were divided into two groups and expertly led by local guides whose knowledge of history and art brought the 18th-century royal palace to life. We passed through an enfilade of grand rooms decorated with gilded mirrors, glittering chandeliers, and rococo furniture. It was easy to imagine the bewigged and powdered gentry inhabiting the halls, playing the harpsicord and strolling the ornamental gardens and parterres. The tour concluded with refreshments – including Portugal’s famous custard tarts – in the Cozinha Velha where the huge chimney dominating the room was easily large enough to roast a whole ox.